Color online) (a) Valence-electron screening cloud around the excited... | Download Scientific Diagram
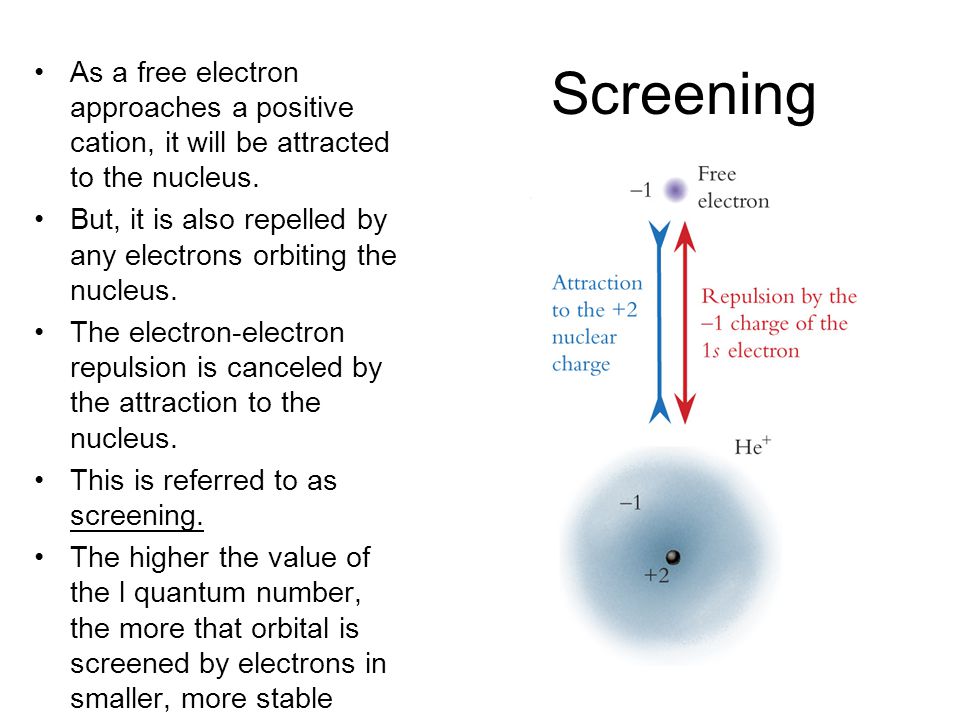
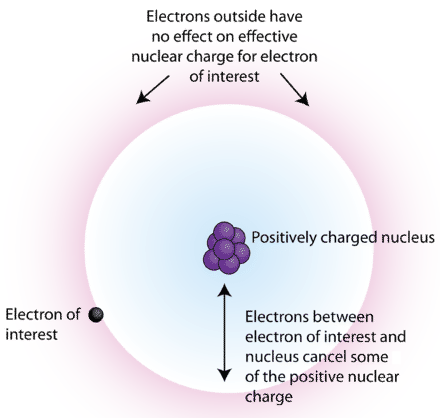
Screening As a free electron approaches a positive cation, it will be attracted to the nucleus. But, it is also repelled by any electrons orbiting the. - ppt download
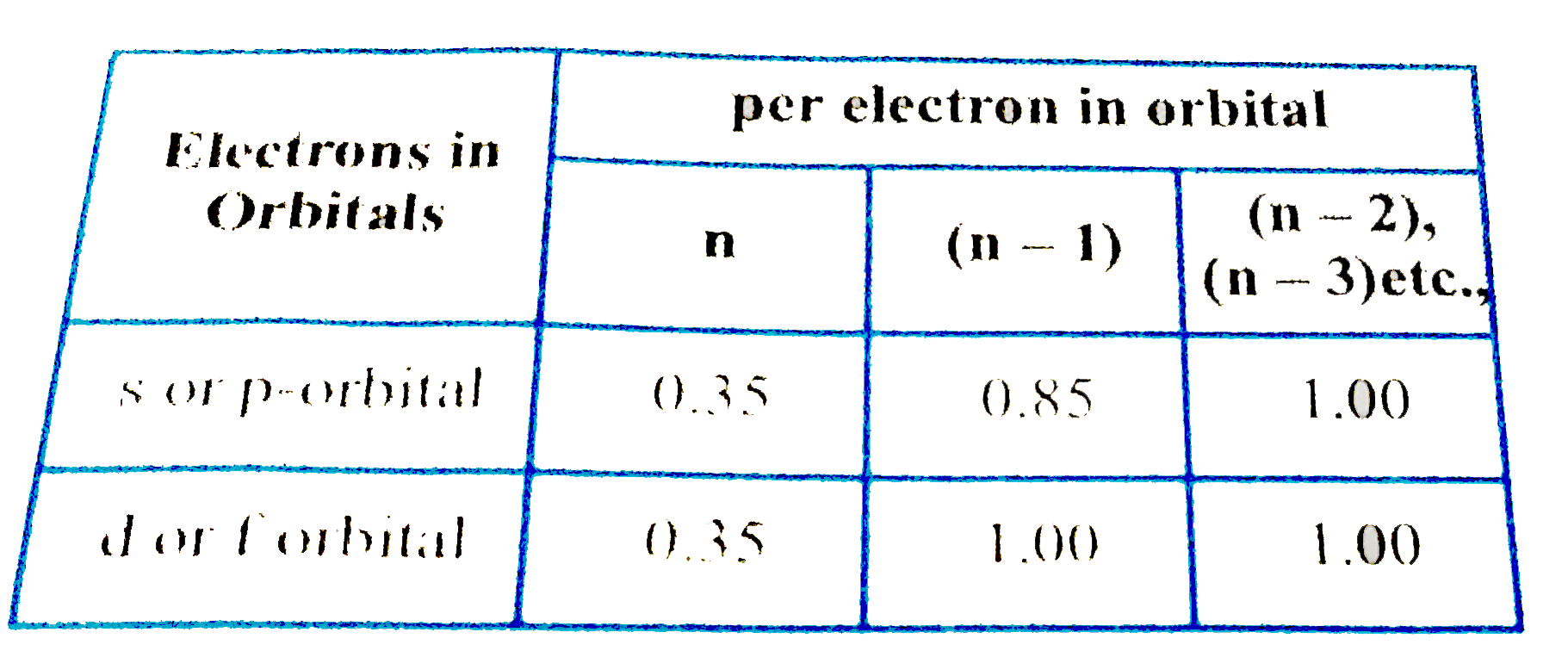
According to I.C slater effective nuclear charge, Z^(**), due to screening, is not exactly equal to the actual nuclear charge Z of the nucleus of the atom. Z^(**) depends on the type

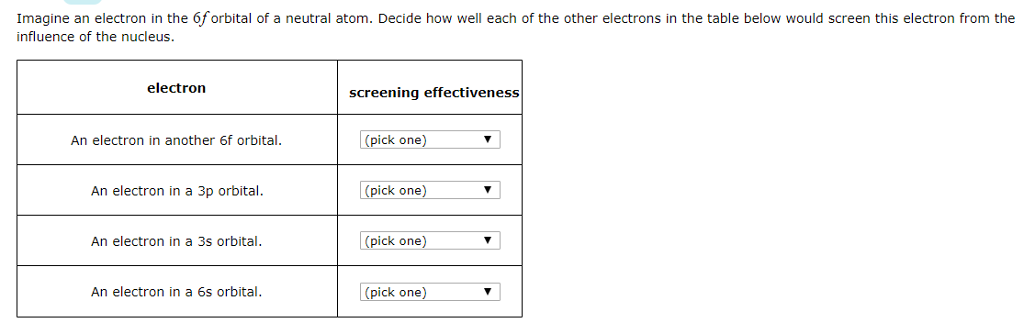
SOLVED: Imagine an electron in the 5d orbital of neutral atom Decide how well each of the other electrons in the table below would screen this electron from the influence of the

PDF) Electron screening effect in the reactions 3He(d, p) 4He and d( 3He, p) 4He Supported in part by INFN, BMBF (06BO812), DFG (436UNG113-146) and OTKA (T025465 | Matthias Junker -

Repulsive electron-electron interaction and nuclear charge screening: Ground state of two-electron atoms: Ndinya, Boniface, Akeyo, Joseph: 9783846540688: Amazon.com: Books
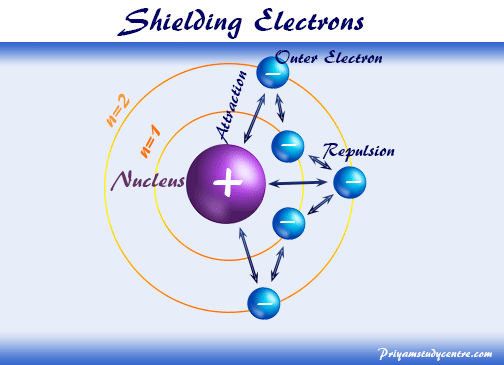
Welcome to Chem Zipper.com......: Effective Nuclear charge (Z* or Zeff): Slater's rule: Screening effect or Shielding effect

SOLVED: Question 37 (1 point) Screening of the nuclear charge by core electrons in atoms is: less efficient than by valence electrons more efficient than by valence electrons essentially identical to that